Understanding the Periodic Table: A Guide for Class 6 to 10 Students
The periodic table might look like a big, confusing grid of letters and numbers, but once you understand how it works, it's like a secret code that unlocks the world of chemistry! Let's explore the periodic table step by step.
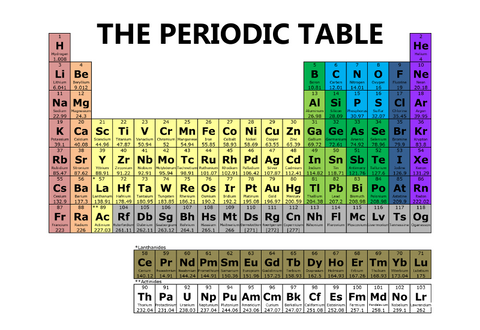
What is the Periodic Table?
The periodic table is a chart that organizes all the chemical elements in a way that shows how they relate to each other. Imagine it as a big family tree, where each element (like Oxygen or Gold) has its special place based on its properties.
The Basics of the Periodic Table
Elements and Symbols:
- Every element has a name and a symbol, which is usually one or two letters. For example, Hydrogen is H, and Sodium is Na.
- The first letter is always capitalized, and if there's a second letter, it's lowercase.
Rows and Columns:
- The rows are called periods. There are 7 periods in the periodic table.
- The columns are called groups or families. There are 18 groups.
- Elements in the same group have similar properties, like how brothers and sisters might have similar traits.
Atomic Number:
- Each element has a unique atomic number, which tells you how many protons are in its nucleus. This number is usually found above the element's symbol.
- For example, Hydrogen has an atomic number of 1, which means it has 1 proton.
Metals, Nonmetals, and Metalloids:
- Metals are usually shiny, good conductors of electricity, and can be shaped easily. Most elements in the periodic table are metals.
- Nonmetals are not shiny and are poor conductors. They are found on the right side of the table.
- Metalloids have properties of both metals and nonmetals and are found between them in the table.
How to Read the Periodic Table
Identify the Element:
- Look at the symbol and name of the element to know what you're dealing with. For example, "O" stands for Oxygen.
Check the Atomic Number:
- The atomic number tells you how many protons the element has. For example, Carbon has an atomic number of 6, so it has 6 protons.
Understand the Groups and Periods:
- Elements in the same group (column) often behave similarly. For example, the elements in Group 1 are all very reactive metals.
- Moving from left to right across a period, elements change from metallic to non-metallic.
Learn About Special Groups:
- Group 1: The Alkali Metals, like Sodium (Na), are very reactive.
- Group 17: The Halogens, like Chlorine (Cl), are also very reactive but are nonmetals.
- Group 18: The Noble Gases, like Neon (Ne), are very stable and don't react much.
Fun Facts About the Periodic Table
- The periodic table was created by a Russian scientist named Dmitri Mendeleev in 1869.
- The table is still growing! New elements are discovered and added every few years.
- The periodic table is organized so that you can predict the properties of elements even if you've never heard of them before.
Conclusion
The periodic table might seem complex at first, but it's like a treasure map for chemists. By understanding the layout and the meaning behind the symbols and numbers, you'll be able to explore the world of elements with confidence. Keep this guide handy, and soon you'll be navigating the periodic table like a pro!

:max_bytes(150000):strip_icc():format(webp)/what-are-the-first-20-elements-608820-FINAL-5b758ab446e0fb002c67279a.png)